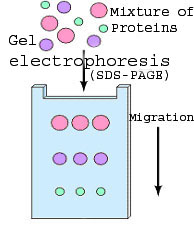
| 1. | A protein sample is subjected
to electrophoresis on an SDS-polyacrylamide gel. This
method separates the various proteins in the sample according to their
size. The sample can be a whole cell lysate or a fractionated
sample following differential centrifugation, immunoprecipitation etc.
In the following virtual experiment the sample is lysed food
vacuoles (examine detailed procedure).
Watch a movie describing SDS-polyacrylamide gel electrophoresis. |
 |
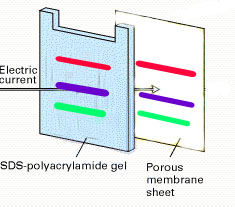
| 2. | Electroblotting transfers the separated proteins from the gel to the surface of a nitrocellulose membrane. Thus, the nitrocellulose membrane is imprinted with the same protein bands as the gel, and the transferred proteins are more accessible for further treatment. |
 |
| 3. | The blot is incubated with a generic protein (such as milk proteins or BSA) which binds to any remaining sticky places on the nitrocellulose. This helps to minimize background signals. |
|
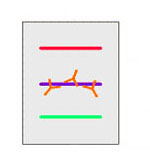
| 4. | An antibody that is specific for the protein of interest (the primary antibody - Ab1) is added to the nitrocellulose sheet and reacts with the antigen. Only the band containing the protein of interest binds the antibody, forming a layer of antibody molecules (but its position can't be seen at this point). |
 |
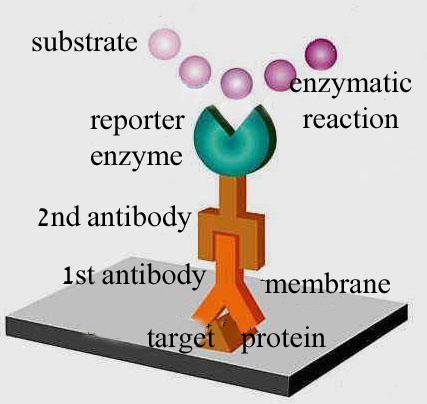
| 5. | Following several rinses
for removal of non-specifically bound Ab1, the Ab1-antigen
complex on the nitrocellulose sheet is incubated with a second antibody
(Ab2), which specifically recognizes the Fc
domain of the primary antibody and binds it. Ab2 is radioactively
labeled, or is covalently linked to a reporter enzyme. This
enables us to derect the protein-Ab1-Ab2 complex
.
|
 |