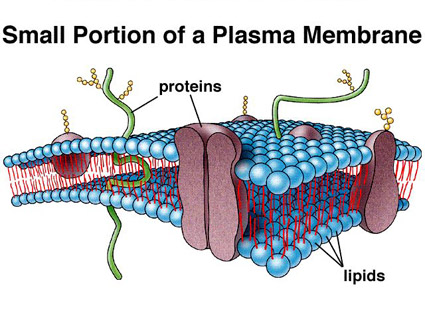
A fluid mosaic
The basic composition and
structure of the plasma membrane is the same as that of the
membranes that surround
organelles and other subcellular compartments. The
foundation is a phospholipid bilayer, and the membrane as a
whole is often described as a fluid mosaic – a
two-dimensional fluid of freely diffusing lipids, dotted or
embedded with proteins, which may function as channels or
transporters across the membrane, or as receptors. The model was
first proposed by S.J. Singer (1971) as a lipid protein model
and extended to include the fluid character in a publication
with G.L. Nicolson in "Science" (1972).
Some of these proteins simply
adhere to the membrane (extrinsic or peripheral
proteins), whereas others might be said to reside within it or
to span it (intrinsic proteins – more at
integral membrane protein). Glycoproteins have
carbohydrates attached to their extracellular domains. Cells may
vary the variety and the relative amounts of different lipids to
maintain the fluidity of their membranes despite changes in
temperature.
Cholesterol molecules (in case of eukaryotes) or
hopanoids (in case of prokaryotes) in the bilayer assist in
regulating fluidity.
Detailed structure
Phospholipid molecules in the
cell membrane are "fluid," in the sense of free to diffuse and
exhibit rapid lateral diffusion.
Lipid rafts and
caveolae are examples of cholesterol-enriched microdomains
in the cell membrane. Many proteins are not free to diffuse. The
cytoskeleton undergirds the cell membrane and provides
anchoring points for integral membrane proteins. Anchoring
restricts them to a particular cell face or surface – for
example, the "apical" surface of
epithelial cells that line the
vertebrate
gut – and limits how far they may diffuse within the
bilayer. Rather than presenting always a formless and fluid
contour, the plasma membrane surface of cells may show
structure. Returning to the example of epithelial cells in the
gut, the apical surfaces of many such cells are dense with
involutions, all similar in size. The finger-like projections,
called microvilli, increase cell surface area and
facilitate the absorption of molecules from the outside.
Synapses are another example of highly-structured membrane.
New material is incorporated
into the membrane, or deleted from it, by a variety of
mechanicsms. (i) Fusion of intracellular vesicles with the
membrane not only excretes the contents of the vesicle, but also
incorporates the vesicle membrane's components into the cell
membrane. The membrane may form blebs that pinch off to become
vesicles. (ii) If a membrane is continuous with a tubular
structure made of membrane material, then material from the tube
can be drawn into the membrane continuously. (iii) Although the
concentration of membrane components in the aqueous phase is low
(stable membrane components have low solubility in water),
exchange of molecules with this small reservoir is possible. In
all cases, the mechanical tension in the membrane has an effect
on the rate of exchange. In some cells, usually having a smooth
shape, the membrane tension and area are interrelated by elastic
and dynamical mechanical properties, and the time-dependent
interrelation is sometimes called homeostasis, area regulation
or tension regulation.
Transport across membranes
As a lipid bilayer, the cell
membrane is semi-permeable. This means that only some molecules
can pass unhindered in or out of the cell. These molecules are
either small or
lipophilic. Other molecules can pass in or out of the cell,
if there are specific transport molecules.
Depending on the molecule,
transport occurs by different mechanisms, which can be separated
into those that do not consume energy in the form of
ATP (passive transport) and those that do (active
transport).
Passive transport
Passive transport is a means
of moving different chemical substances across membranes through
diffusion of
hydrophobic (non-polar) and small polar molecules, or
facilitated diffusion of polar and ionic molecules, which relies
on a
transport protein to provide a channel or bind to specific
molecules. This spontaneous process decreases free energy, and
increases entropy in a system. Unlike active transport, this
process does not involve any chemical energy (ATP).
Active transport
Typically moves molecules
against their
electrochemical gradient, a process that would be
entropically unfavorable were it not
stoichiometrically coupled with the hydrolysis of ATP. This
coupling can be either primary or secondary. In the primary
active transport, transporters that move molecules against their
electrical/chemical gradient, hydrolyze ATP. In the secondary
active transport, transporters use energy derived from transport
of another molecule in the direction of their gradient, to move
other molecules in the direction against their gradient. This
can be either
symport (in the same direction) or
antiport (in the opposite direction).
Examples include:
-
The usual cases of
molecular exchangers,
transporters and
pumps
-
endocytosis and
exocytosis, where molecules packaged in membrane
vesicles are either imported or exported respectively,
can be thought of as active transport.