Experimental Procedure
Rts reactions (50 ul) were run
for 6 hours. Binding buffer (450 ul) was added to each sample, and samples
were than incubated with gluthatione beads for one hour. Samples were than
centrifuged and the supernatant was collected. These sup samples are the
Unbound
fraction. Samples were washed once more with the binding buffer and sample
buffer was than added. These are the Bound fractions of each sample.
We used His-GFP (supplied by the manufacturer) as
a control system. These samples were run on different gels, since different
antibodies were used for detection. These results are not shown here.
Scroll down to see ALL
results.
|
Conditions
|
Coomassie Staining
|
Western Blot (anti-GST
ABs)
|
Experimental Conditions
|
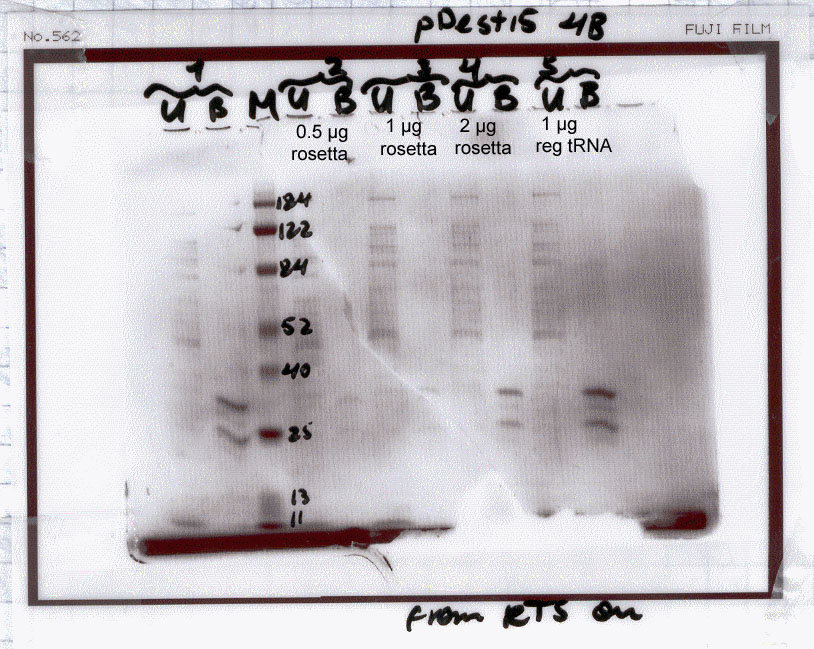
| NS4B wt |
 |
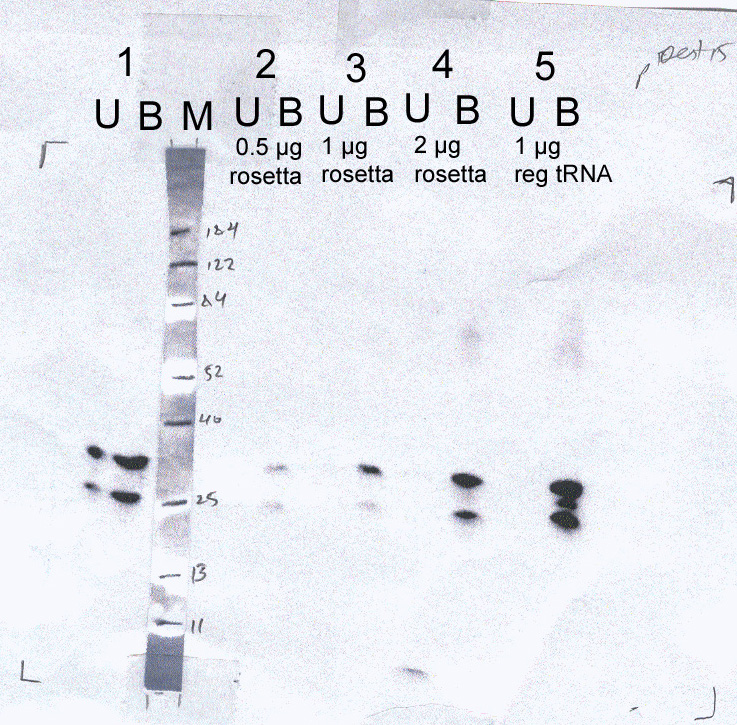 |
1 - no supplements
2 - + 0.5 ug tRNAs derived
from Rosetta.
3 - + 1.0 ug tRNAs derived
from Rosetta.
4 - + 2.0 ug tRNAs derived
from Rosetta.
5 - + 1.0 ug tRNAs derived
from a regular strain (this is a control sample used to show whether
tRNAs supplement affects protein synthesis in this system). |
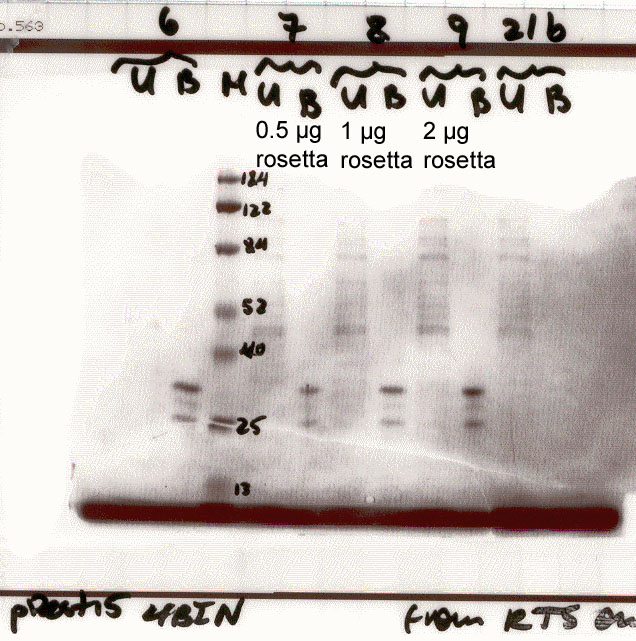
| NS4B mutant |
 |
 |
6 - no supplements
7 - + 0.5 ug tRNAs derived
from Rosetta.
8 - + 1.0 ug tRNAs derived
from Rosetta.
9 - + 2.0 ug tRNAs derived
from Rosetta.
21b - no template DNA was
added to this reaction. |
.
Q6
How
can we interpret these results?
Read
ALL answers and choose between "right" and "wrong".
There
may be more than one "right" answer.
1.
The protein products are fully bound by the glutathione
beads, indicating their solubility.
2.
Under all experimental conditions smaller products
than expected are synthesized.
3.
Addition of tRNAs derived from Rosetta cells improves
the quality and the quantity of the synthesized protein.
4.
In the absence of template DNA de-novo protein synthesis
does not occur.
5.
The mutant gives somewhat higher yields of protein
than the WT, though the products are shorter than expected as observed
in the WT.
Have
you examined all possibilities?
Continue.



