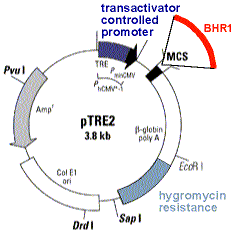
| Now you transfect these
cells (which already contain the tetracyclin transactivator)
with a plasmid containing the BHR1 gene under control of the tetracyclin
controlled promoter. The plasmid you use confers resistance to hygromycin.
|
 |
| Now you transfect these
cells (which already contain the tetracyclin transactivator)
with a plasmid containing the BHR1 gene under control of the tetracyclin
controlled promoter. The plasmid you use confers resistance to hygromycin.
|
 |
Following transfection you start a seven days selection period in the presence of G-418 + hygromycin. At the end of this period you have to determine which clones incorporated the BHR1 gene into their genome and now express it on their plasma membrane. You will do that in two different ways:
Your conclusions are (choose between right and wrong):
You now proceed with the
second assay - using an anti BHR1 antibody to detect the receptors in intact
cells.
Each of the same four cell-lines
was plated into two different dishes. A day later you added tet to one
well from each cell line and added only the tet buffer (blank reagents)
to the other well. After two more days you exposed the cells to mouse
anti BHR1 antibody (=primary antibody) and then to goat anti mouse antibody
(=secondary antibody) labeled with rodamine (red fluoresence) and examined
the cells in a confocal microscope. The results are presented below (Click
on any clone number to get more details).
|
The following conclusions
are based on results obtained using the two procedures.
1.
In Noam-10-1 clone the expression is leaky, and that is why there is no
difference between induced and non-induced cells.
|
Did you try working with the baculovirus expression system?