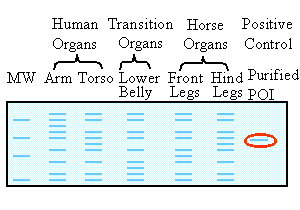
| 1. Wrong
- we can easily recognize the purified POI (our positive control) in the
last sample to the right, where it is the only protein in the sample (circled
in red). Indeed, all other samples contain bands of
simillar size. However, these bands can be positively identified as POI,
only if they are recognised by the POI specific antibody, used in the Western
blot (lower panel). Since these bands are not
recognized by the POI specific antigen, they are unrelated to POI though
they have a simillar size. Indeed, whole cell lysates contain numerous
proteins of various quantities, some of which may be of the same size.
Commassie staining is less sensitive than immunoblotting. It detects higher amounts of ALL proteins. |
 |